 Periodic Table
Periodic Table
Step I. Speculate on the
following
Ø What do you know about the periodic table? What is it?
Ø What do you know about inventor of the periodic table?
Ø What role does the periodic table play in chemistry?
Ø Do you think the invention of the periodic table is a
great deal? Why?
Step II. Find in the text
sentences with the following word combinations and try to guess their meaning:
Ø
tabular
arrangement, recurring trends, useful framework, rudimentary tables, wide
application.
Step III. Match the following:
|
1. присоединить |
a. ubiquitous |
|
2.
включать |
b. incorporate |
|
3. составлять |
c. comprise |
|
4. короткий сон |
d. draw up |
|
5. распространенный |
e. nap |
Text
The Periodic
Table of the Chemical Elements
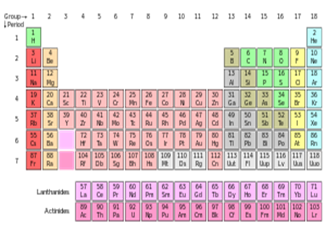 The periodic table is a
tabular arrangement of the chemical elements, organized on the basis of their atomic numbers, electron
configurations, and recurring chemical properties. Elements are presented in order of increasing atomic number (number of
protons). The standard form of the table comprises an 18-column-by-7-row main
grid of elements, with a double row of elements below. The rows of the table are called periods; the columns are called groups, with some of these having names such as halogens or noble gases.
The periodic table is a
tabular arrangement of the chemical elements, organized on the basis of their atomic numbers, electron
configurations, and recurring chemical properties. Elements are presented in order of increasing atomic number (number of
protons). The standard form of the table comprises an 18-column-by-7-row main
grid of elements, with a double row of elements below. The rows of the table are called periods; the columns are called groups, with some of these having names such as halogens or noble gases.
Since, by definition, a periodic table
incorporates recurring trends, any such table
can be used to derive relationships between the properties of the elements and
predict the properties of new, yet to be discovered or synthesized, elements.
As a result, a periodic table provides a useful framework for analyzing
chemical behavior.
All elements from atomic numbers 1(hydrogen) to
119 (ununennium) have been discovered or synthesized. The elements from 1 to 98
(californium) have been found
to exist naturally, although some have
been discovered by synthesis in laboratories. The elements after 98 have
only been synthesized in laboratories.
The invention is credited to
Russian chemist D.I. Mendeleev in 1869. Mendeleev set
down his first ideas at breakfast. He drew up several rudimentary tables, and
then made 63 cards, one for each of the known elements. On each card he put the
properties of the element that he thought most important. He juggled the cards
until he had an arrangement that satisfied him, wrote it down, and went to bed.
He awoke from his nap with the idea that he should arrange the elements in
vertical rather than horizontal groups and transposed them accordingly.
The periodic table is now ubiquitous within the
academic discipline of the chemistry, providing an extremely useful framework
to classify, systematize, and compare all of the many different forms of
chemical behavior. The table has found wide application in chemistry, physics,
biology, and engineering, especially chemical engineering.
Topical vocabulary:
arrange [ə'reɪnʤ] – располагать, расставлять; arrangement – расположение, расстановка; draw up – составлять; framework
– основа; grid – сетка, решетка; halogen – галоген; horizontal [ˌhɔrɪ'zɔnt(ə)l] – горизонтальный; incorporate – включать, объединять; juggle ['ʤʌgl] – жонглировать; nap – короткий сон, дремота; predict – предсказать; synthesis
['sɪnθəsɪs] – синтез; synthesize ['sɪnθəsaɪz]
– синтезировать; tabular [ 'tæbjulə] – табличный; transpose – переставлять, перемещать; ubiquitous [juː'bɪkwɪtəs] – повсеместный.
Step IV.
Translate the following word combinations from English into Russian.
halogens and noble gases_____________________________________
by definition_______________________________________________
chemical behaviour__________________________________________
exist naturally_______________________________________________
the invention is credited to_____________________________________
set down his ideas___________________________________________
juggle the cards_____________________________________________
he transposed them accordingly ________________________________
provide an extremely useful framework__________________________
chemical engineering_________________________________________
Step V. Complete the following sentences.
1. Elements in the periodic table are presented …
2. The Periodic table includes …. columns and …. rows.
3. Mendeleev wrote his first ideas ….
4. Mendeleev thought he should arrange the elements …
5. The table is applied not only in chemistry but in
such closely related sciences as ….
Step VI. Agree or disagree.
1. The
periodic table is a table of the chemical elements in which the elements are
arranged in order of increasing atomic weight.
2. The standard form of the table
includes periods (usually horizontal in the periodic table) and groups (usually
vertical).
3. All the elements in
the periodic table have been found to exist naturally.
4. The periodic table was
invented by D.I. Mendeleev in 1870.
5. Mendeleev had an idea
he should arrange the elements in horizontal rather than vertical groups.
6. The invention of the
periodic table is an astonishing achievement within chemical sciences.
Step VII. Match the words with their synonyms.
|
1. comprise |
a. represent |
|
2.present |
b. contain |
|
3. incorporate |
c. widespread |
|
4. recurring |
d. include |
|
5. framework |
e. periodic |
|
6.invention |
f. base |
|
7. ubiquitous |
g. idea |
Step VIII. Answer the following questions
1. Can you give the definition of the periodic table?
2. How many periods and groups does the periodic table
comprise?
3. Does the periodic table predict the properties of
the elements?
4. Have all the elements in the periodic table been
synthesized in laboratories?
5. Why is the periodic table ubiquitous within the
academic discipline of the chemistry?
6. What other closely related sciences has the
periodic table wide application in?
7. Why did Mendeleev leave gaps in the periodic table
of elements?
Step IX. Read
the dialogue and answer the question
v What do you think of the discovery? Is it possible?
Can you prove your opinion?
Dialogue
MIKE: Ann. I've heard that a new element was discovered by
one of our fellow students. Can you imagine that!
ANN: Hm... What kind of element is it?
MIKE:As far as I know, it's a transuranium element.
ANN: Was it given any number in the Periodic Table?
MIKE: Yes, its number is 114. Leo said that this
discovery...
ANN: Leo? Leo who?
MIKE: Leo Matveev. He is the student who found the origin of
the element.
ANN: Matveev. How did he do it?
MIKE: I'm trying to tell you, but you interrupt me all the
time.
ANN: I'm sorry. Go ahead, please.
MIKE: The thing is, Leo thinks that this discovery was
predicted by Leonardo da Vinci as early as the 16th century.
ANN: How did Leo find it out?
MIKE: guess, he's
read Leonardo da Vinci's famous book Anatomy.
ANN: Yes, and so what?
MIKE: Well, according to Leo, da Vinci gave a column of
digits and didn't tell anybody about their meaning.
ANN: Do you remember these figures?
MIKE: Yes. Here you are. Vertical column with digits: 8, 7,
1, 7, 4, 7. Leonardo da Vinci was crazy about coding his ideas. But our Leo supposed that this column
contains some information which was dangerous for those times. Or probably too
early to decipher it.
ANN: More than that, these were the times of alchemy and
all scholars were trying to discover the formula of "philosophic
stone".
MIKE: Correct. And our fellow student made an attempt to
decipher the column by means of simple arithmetic operations.
ANN: Could you show me his calculations?
MIKE: I'm afraid I can't. I remember only the result. Two
numbers were obtained: 114 and 184.
ANN: I see. It's a formula of this element.
MIKE: Quite right. Our Leo has once again proved the genius
of the great Italian.
ANN: What about the name of the element? Does any name
exist?
MIKE: Yes, believe me or not, Leo suggested the name of the
element in honour of Leonardo da Vinci — Leonardium.
ANN:
Only
in honour of da Vinci?...
Step
X. Write the names.
1. _______________ discovered a new
element.
2.
Ann is interrupting
________________________all the time.
3. The discovery of the element was
predicted by _______________.
4. __________________were trying to
discover the formula ‘philosophic stone’.
5. ______________is doubting in naming of
the element in honour of Leonardo da Vinchi.
Step
XI. Answer the following questions:
1. Who found the information about the
number 114 element?
2. Who predicted the discovery of the
element?
3. When was the discovery predicted?
4. What famous book by Leonardo da Vinci
did Leo read?
5. How did Leonardo da Vinci predict the
element?
6. What special was about the great man?
7. What was one of the reasons for coding
the discovery?
8. Who suggested the name of the element?
9. What does the last line in the dialogue
mean?
10. If you
discovered an element, what would you name it?
Step XII.
Discuss with your partner which you think are the most important scientific
discoveries of the past. Say what you think and find out if your partner agrees
or disagrees with you.
Step XII.
Prepare a short report about the development of the periodic table and
contributions from many famous chemists and other eminent scientists.

Fun
Time
Periodic
table quiz
1. Which element derives its symbol from the Latin word,
aurum, which means 'shining dawn'?
A. Aluminum
B. Americium
C. Gold
D. Silver
2. Which element
best characterizes an organic compound?
A. Oxygen
B. Carbon
C. Hydrogen
D. Nitrogen
3. Which element is an example of a noble gas?
A. Krypton
B. Chlorine
C. Iridium
D. Nitrogen
4. This one is intended to be a confidence builder.
These are diamonds. Diamonds are pure:
 A. boron
A. boron
B. carbon
C. iron
D. nitrogen
E. oxygen
 5.You
may encounter this element in pure form fairly often. This metallic element is:
5.You
may encounter this element in pure form fairly often. This metallic element is:
A.brass
B. bronze
C. copper
D. gold
E. zinc
6.This
metallic element is a liquid at room temperature. It is:
 A.bromine
A.bromine
B. iodine
C. iron
D. lead
E. mercury
7.This
yellow element is a component of gunpowder. It is:

A.chlorine
B. copper
C.gold
D. selenium
E. sulfur
 8. You
usually encounter this element as a gas, though it is possible to find it in
liquid form. You aren't likely to see the solid except in this photo. It is:
8. You
usually encounter this element as a gas, though it is possible to find it in
liquid form. You aren't likely to see the solid except in this photo. It is:
A. chlorine
B.
helium
C.
hydrogen
D. nitrogen
E.
radon
9. All of the following are
alkali metals, except which element?
A. Sodium
B. Rubidium
C. Cesium
D. Radium