 Chemical
Compounds
Chemical
Compounds
Step I. Speculate on the
following
Ø
What’s
a compound?
Ø
Are
there any kinds of chemical compounds? What kinds of chemical compounds do you
know? Name them.
Ø
What
kind of chemical compound is extremely common on the Earth?
Step II. Find in the text
sentences with the following word combinations and try to guess their meanings:
Ø
decomposition
temperature; general scientific usage; general term; molten salts; with
exception of.
Step III. Match the following:
|
1. химическое
соединение |
a. decomposition |
|
2.
определять |
b. refer to |
|
3.
разложение |
c. chemical compound |
|
4.
раствор |
d. determine |
|
5.
реакция нейтрализации |
e. solution |
|
6.
относиться к чему-либо |
f.
neutralization reaction |
Text
Chemical Compounds
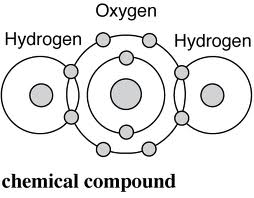 A
chemical compound is a chemical substance formed from two or more elements,
with a fixed ratio determining the composition.
A defining characteristic of a compound is that it has a chemical
formula. Formulas describe the ratio of atoms in a substance, and the number of
atoms in a single molecule of the substance. The formula does not indicate that
a compound is composed of molecules; for example, sodium chloride (table salt,
NaCl) is an ionic compound. Compounds may have a number of possible phases. Most
compounds can exist as solids. All compounds can decompose to smaller compounds
or individual atoms if one heats to a certain temperature (called the
decomposition temperature).
A
chemical compound is a chemical substance formed from two or more elements,
with a fixed ratio determining the composition.
A defining characteristic of a compound is that it has a chemical
formula. Formulas describe the ratio of atoms in a substance, and the number of
atoms in a single molecule of the substance. The formula does not indicate that
a compound is composed of molecules; for example, sodium chloride (table salt,
NaCl) is an ionic compound. Compounds may have a number of possible phases. Most
compounds can exist as solids. All compounds can decompose to smaller compounds
or individual atoms if one heats to a certain temperature (called the
decomposition temperature).
There are the
following types of compounds: acids, bases, ionic compounds, salts, oxides,
organic compounds and so on.
An acid is
typically a water-soluble, sour-tasting chemical compound. In common usage an
acid is a species that, when dissolved in water, gives a solution with a pH of
less than 7. In general scientific usage an acid is a molecule or ion that is
able to give up a proton to a base, or accept an unshared pair of electrons
from a base. An acid reacts with a base in a neutralization reaction to form a
salt.
Bases and acids
are referred to as opposites because the effect of an acid is to increase the
hydronium ion concentration in water, whereas bases reduce this concentration.
An ionic
compound is a chemical compound in which ions are held together in a lattice
structure by ionic bonds. To form an ionic compound, there needs to be at least
one metal and one non-metal. The metal element is usually the positive charge
and the non-metal element is a negative charge. Some properties of ionic
compounds are high melting and boiling points and good conductivity. Ionic
compounds are solids at room temperature and will usually form crystals.
Salt is a
general term used for ionic compounds composed of positively charged cations
and negatively charged anions, so that the product is neutral and without a net
charge. Solutions of salts in water are called electrolytes. Electrolytes as
well as molten salts conduct electricity. Zwitterions are salts that contain an
anionic center and a cationic center in the same molecule.
An oxide is a
chemical compound of oxygen with other chemical elements. Oxides are extremely
common in Earth's crust, and indeed in solid matter throughout the universe.
Oxides are usually created through the process of oxidation. Generally, oxides
are not conductive to electricity.
An organic
compound refers to any member of a large class of chemical compounds whose
molecules contain carbon, with exception of carbides, carbonates and carbon
oxides. The study of organic compounds is termed organic chemistry.
Topical vocabulary
acid –кислота; base – основание; carbide
['kɑːbaɪd] – карбид; carbonate
['kɑːb(ə)neɪt] – карбонат; conductivity
[ˌkɔndʌk'tɪvətɪ] – проводимость, conduct - проводить; decompose [ˌdiːkəm'pəuz] - разлагать (ся), распадаться; decomposition - разложение, распад; dissolve [dɪ'zɔlv] - растворять (ся); hydronium [haıʹdrəunıəm]
– гидроксоний; lattice structure – решетчатая структура; molten salts – расплав солей;
oxide
['ɔksaɪd] - оксид ; ratio ['reɪʃɪəu] –отношение, соотношение, пропорция; sodium chloride ['səudɪəm]
['klɔːraɪd] - хлорид натрия, поваренная соль; soluble ['sɔljəbl – растворимый; solution
– раствор;
sour ['sauə] – кислый; species
['spiːʃiːz] – вид, разновидность; Zwitterion
[ʹtsvıtəraıən] - цвиттер-ион, амфотерный ион, амфион (биполярный ион) (молекула, которая, являясь в целом электронейтральной, в своей структуре имеет части, несущие как отрицательный, так и положительный заряды).
Step IV. Find in
the text English equivalents to the following Russian word combinations:
xимическое вещество, образованное из двух или более
молекул; основная характеристика соединения; формулы изображают соотношение
атомов в веществе; формула не показывает, что вещество состоит из молекул; растворимое в воде и кислое на вкус
химическое соединение; раствор, ph которого ниже
7; химическое соединение, в котором ионы имеют решетчатую структуру;
температура плавления; температура
кипения; положительный и отрицательный заряды; хорошая проводимость; твердые вещества при комнатной температуре;
положительно заряженные катионы;
отрицательно заряженные анионы; без результирующего заряда; соли,
содержащие катионный и анионный центры в
одной молекуле; соединение кислорода с другими химическими элементами; земная
кора; в процессе окисления; соединения, чьи молекулы содержат углерод.
Step V. Agree or
disagree.
1. A chemical compound is a
chemical substance formed from only one element.
2. A defining characteristic of a compound
is that it has no a chemical formula.
3. Bases and acids are referred to a
similar chemical compounds because the effect of an acid is to increase the
hydronium ion concentration in water, whereas bases reduce this concentration.
4. To form an ionic compound, there needs
to be at least one metal and one non-metal. The metal element is usually a
negative charge and the non-metal element is a positive charge.
5. Ionic compounds are solids at room
temperature and will usually form crystals.
6. Solutions of salts in water are called
electrolytes and molten salts can conduct electricity.
7. Oxides are extremely
common in Earth's crust, and indeed in solid matter throughout the universe.
8. Generally, oxides are conductive to
electricity.
9. An organic compound is a chemical
compounds whose molecules contain carbon.
10. The study of organic compounds is
termed inorganic chemistry.
Step VI. What is
this?
1. A chemical substance formed from two or more
elements is _________________. 2. A representation of a substance using symbols
for its constituent elements is _____________. 3. Water-soluble and
sour-tasting chemical compound is _______________. 4. A product of
neutralization reaction, when an acid reacts with base is _______________. 5. A
chemical compound in which ions are held together in a lattice structure by
ionic bonds is _____________. 6. A liquid or gel that contains ions and can be
decomposed by electrolysis is ______________. 7. Salts including an anionic
center and a cationic center in the same molecule are ________________. 8. A
chemical compound containing two oxygen atoms per molecule is _____________. 9.
A chemical compound containing one oxygen atom and one other element
is________________. 10. Compounds whose molecules contain carbon are
_____________. 11. A compound of chemical elements containing no carbon
atoms are _______________.
Step VII. Answer
the questions.
1. What is a chemical
compound?
2. What describes formula?
3. Can compounds decompose to a smaller compounds
or individual atoms?
4. What types of compounds do you know?
5. What is acid?
6. Are bases opposite to acids?
7. What is an ionic compound?
8. Ionic compounds are solids at room
temperature, aren’t they?
9. What is an oxide?
10. What’s an organic compound?
11. What is the difference between an
element and a compound?
Step VIII.
Discuss with your partner the following statements.
1.
Compounds may
have several possible phases.
2. Physical forces rarely break down compounds
completely.
3. Compounds have few or none of the physical or chemical
traits of the original element.
Step IX. Choose
any chemical compound and prepare a report.

Fun Time
Chemistry Quiz
1. Can an element be broken down into a simpler substance?
A. Yes
B. No
2. What is a compound?
A. A substance
made up of one kind of mixture
B. A substance
made up of one kind of element
C. A substance
made up of two or more elements
3. Sea water, smog and brass are all examples of …?
A. Alloys
B. Ionic compounds
C. Mixtures
D. Covalent compounds.
4. The simplest unit of a compound is?
A. Neutron
B. Proton
C. Atom
D. Molecule
5. What is used to represent the number of atoms of
each element in a compound?
A. Mass number
B. Valence number
C. Atomic number
D. Chemical formula
6. Sodium chloride is also known as
A. Baking soda
B. Table salt
C. Washing soda
D. Bleach
7. Which of the following is a compound?
A. Calcium
B. Oxygen
С. Water
D. Hydrogen
8. Are there more compounds than there are elements?
A. Yes
B. No